What is FRET?
Förster resonance energy transfer (FRET) describes a physical phenomenon of non-radiative energy transfer based on dipole-dipole coupling that can occur from an excited state fluorophore (the donor) to a ground state fluorophore (the acceptor) (Figure 1). FRET is detected by the appearance of sensitized fluorescence in the acceptor and by a decrease (quenching) of donor fluorescence.
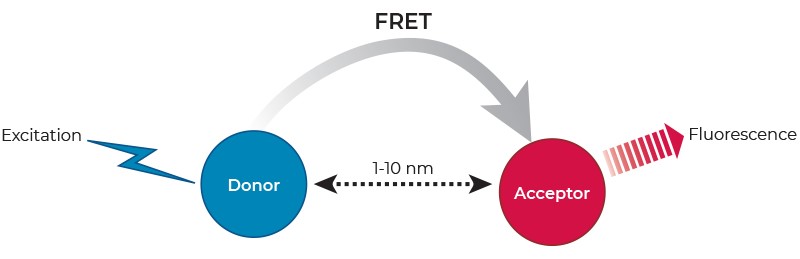
FRET only occurs when the donor fluorescence emission spectrum overlaps with the acceptor excitation spectrum, when donor-acceptor relative dipole orientations are approximately parallel, and when the donor and acceptor are within close proximity (typically 1-10 nm). The latter has led to the widespread application of FRET in biomedical research for the study of molecular interactions, as well as the measurement of distances on a molecular scale.
Since the transfer of energy occurs only when the donor and acceptor are in close enough proximity, FRET based assays are homogeneous. That is, they do not require any washing or separation steps to remove distant donor and acceptor partners. However, the performance of traditional FRET assays using conventional fluorophores is often compromised by the susceptibility of conventional fluorophores to photobleaching, and by scattered light and high background fluorescence from sample components (e.g., cell debris, buffers, test compounds and microplates). This makes FRET signal-to-background (S/B) levels unsatisfactory for many applications.

 Deutsch
Deutsch


