Under current circumstances, PCR tests are mainly associated with identification of COVID-19 infections. However, PCR tests have already been in use for the detection of infectious diseases, such as intestinal infection, CMV, Group A and B Streptococcus or even malaria, for several years now. Various PCR platforms are used to determine the DNA/RNA content of the virus or pathogen. A glimpse into the technology can also be favorable for further improvement in the efficiency of COVID-19 PCR tests.
The polymerase chain reaction (PCR) is the most important method in laboratories to investigate the discrete molecular structure of the genetic material. This consists of deoxyribonucleic acid (DNA) and contains the genetic code for humans as well as for animals and plants. Additionally, also bacteria, fungi, viruses, and parasites encode their genetic information in DNA/RNA. With PCR tests, the DNA/RNA content of pre-processed samples are amplified and used to detect mutations, infections or hereditary diseases.
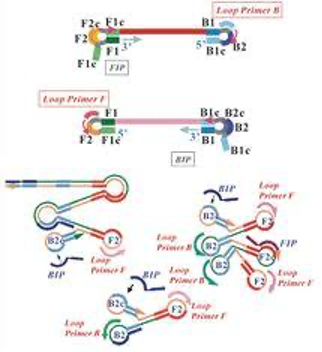
LAMP (LOOP-MEDIATED ISOTHERMAL AMPLIFICATION) TECHNOLOGY
The loop-mediated isothermal amplification (LAMP) technology was developed in Japan by the Eiken Chemical cooperation (Notomi T. et al., 2000) and is used for the detection of several infectious diseases: diarrhea, respiratory viruses, sexually transferable diseases (e.g. fungi infections), Neisseria gonorrheae, HSV-1 and -2 or malaria among others. For the LAMP application, a complementary primer is added to the sample, leading to the formation of a “stem-loop” structure. The amount of the formed side product - magnesium pyrophosphate - during this reaction is measured at the end of the reaction and is proportional to the DNA content of the sample. Results are analyzed qualitatively. As the reaction takes place ISOTHERMALLY at a temperature of 63 °C no conventional thermal cycler is necessary.

Advantages: Incubators, such as alethia®, can process samples from ten patients at once, having the results ready after 40 minutes as read-out, which is comparably faster and more straightforward than conventional PCR tests. Moreover, several infectious diseases can be detected at once using this method.
REVOGENE – Novel RT-PCR (REAL-TIME-POLYMERASE-chain-REAKTION) PLATFORM

Revogene® is a flexible molecular platform, capable of performing single as well as multiplex PCR tests. An advantageous feature is the small set-up area needed for this platform (length: 33 cm, width: 24 cm, depth: 40 cm, weight: 10 kg). Hence, this platform is utilizable in every laboratory. Revogene® can be used to detect nosocomial infections, cause of diarrhea, Group A and B Streptococcus or for Carba C assays, delivering reliable results within 70 minutes. For this methodology, samples are loaded onto the test cassette (PIE) which is put into the machinery prior to the test.
Advantages: It is possible to detect 12 different target sequences per sample with a capacity of 8 samples per run. The parameters can be adjusted accordingly to use as the PCR protocols are identical. Test results are ready after 42-70 minutes.
For sample preparation only a few steps are necessary:

PIE = test-cassette containing all reagents for the RT-PCR run.

1: Sample Loading chamber; 2: Homogenization chamber; 3: Overflow chamber;
4: Dilution/Lysis chamber; 5: Three PCR wells (#1 to #3 at the left) and one waste chamber (at the right)
The graphical interface of Revogene® is intuitive and easy to operate. With the bidirectional communication function, it is possible to utilize this system in every laboratory.
Outlook
These examples demonstrate that PCR tests can also be performed with smaller sized equipment and minimal effort. At the moment, detection of COVID-19 still requires elaborate laboratory equipment as well as trained staff. However, the progress has not stopped in this regard either.

 Deutsch
Deutsch


