Our immune system does not only consist of antibodies and lymphocytes. The so-called complement system is part of the innate, non-specific immune system and involves more than 40 plasma proteins, which are only activated upon infection. On the one hand, the complement factors protect the non-infected neighbouring cells and limit the spread of the inflammation, on the other hand, they support the combat of bacteria and viruses by stimulating proinflammatory reactions, such as the release of histamine or the activity of the scavenger cells and killer cells.
A restricted complement activity leads to a more sensitive reaction towards repetitive violent or severe infections in humans and could impact the development of autoimmune diseases. An inadequate activation of the complement causes chronic inflammation as well as tissue damage. Particularly in case of autoimmune diseases, operations, severe injuries or immunosuppressive therapies, a complement defect could increase the risk for the patient and complicate therapy. This makes complement diagnostics all the more important – not only in case of acute suspicion, but also as a precautionary measure to identify potential risk patients.
But which factors are responsible when the complement system is not functioning properly, as it is not or not sufficiently activated? For this, it is worth to take a glance on how the activation actually works and which chain reaction it triggers.
How is the complement system activated?
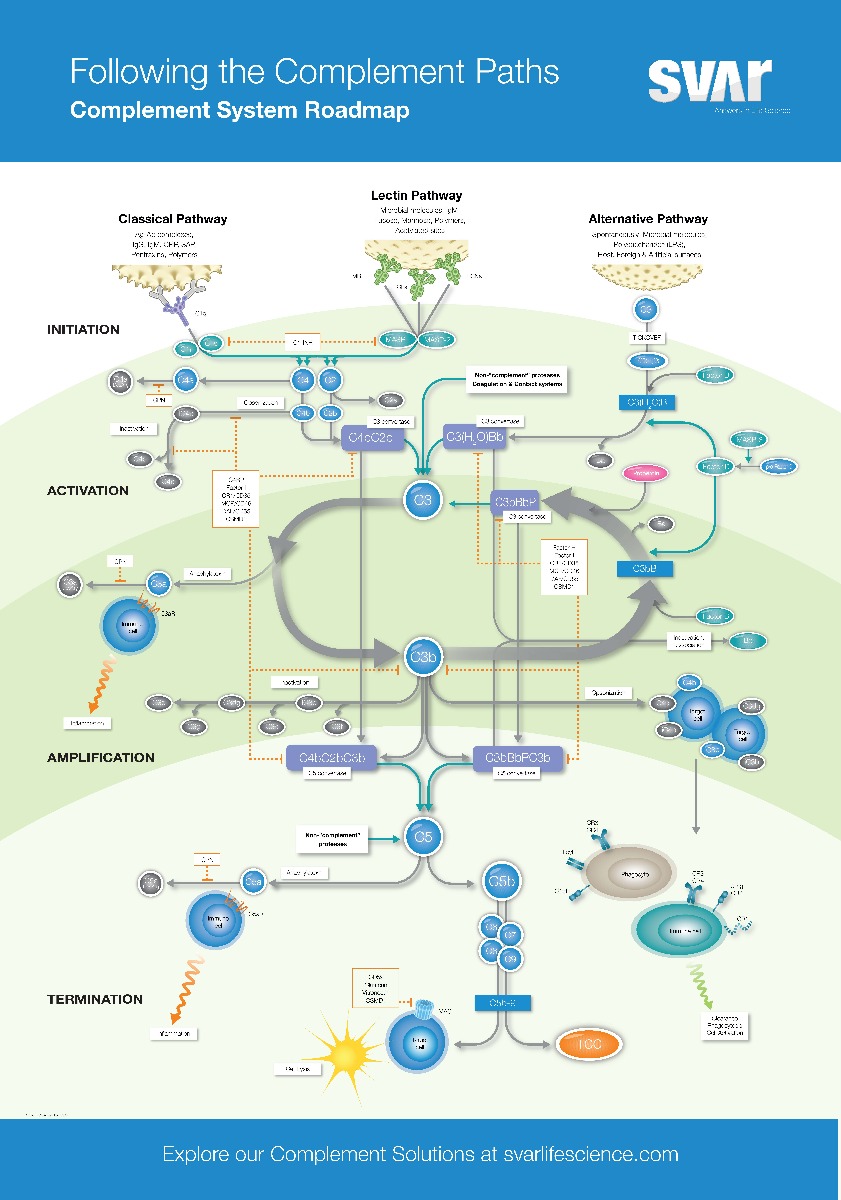
There are three different pathways of activation: the classical, the alternative and the later discovered MBL (mannose-binding lectin) pathway, mostly called the lectin pathway. All three activation pathways trigger a chain reaction with the aim to recognise and fight pathogens quickly. During this process, the proteins of the complement systems are split in smaller fragments. Key is the formation of the enzyme complex C3-convertase, which cleaves C3 in C3a and C3b. C3b binds to the surface of the bacteria or viruses and activates further complement factors. By this, the inflammation reaction is started and the pathogen is marked, making it better recognisable to immune cells, such as macrophages and neutrophil granulocytes, for phagocytosis.
Additional to marking of the pathogen by C3b the complement system is also involved in the direct destruction of the pathogen. C5 is also cleaved by the C3-convertase into C5a and C5b. C5b binds to the surface of the pathogen and recruits other proteins to form the membrane attack complex (MAC). The MAC induces the formation of pores in the membrane of the pathogen which then leads to cell death.
The classical pathway via antibodies
The classical complement pathway is activated by antibodies which bind specifically to pathogenic molecules. As soon as an antibody is bound to a bacteria or virus, it can activate the complement system by binding to the first enzyme of the cascade, C1. By this, the chain reaction described above is started.
The lectin pathway
In contrast to the classical pathway, the lectin pathway is not activated by antibodies but by lectin, which binds to the pathogenic molecules.
Among those lectins is MBL (mannose-binding lectin), which attaches to carbohydrates on the surface of the bacteria and viruses. As soon as the MBL is bound to carbohydrates of the pathogen, it is activated and forms a complex with other proteins, the MBL-associated serine protease (MASP). This complex cleaves proteins and induces the activation of the complement system.
The alternative complement pathway
The alternative pathway works – differently to the classical and the lectin pathway – independently of antibodies and lectins. Instead, the alternative pathway is maintained by a constant low-level activation of complement proteins in the blood plasma.
The alternative signalling pathway starts with a spontaneous cleavage of C3 in C3a and C3b and triggers the reaction cascade with this.
Which roles do factor P (properdin) and factor H play in the alternative signalling pathway?
The constant low-level activation of the alternative signalling pathway is regulated by complement proteins, such as factor H and I, which prevent excessive activation of the complement system towards endogenous. Factor H binds to C3b and inhibits its activation whereas factor I inactivates C3b.
Properdin is a regulatory protein which enhances and stabilises the activation of the alternative signalling pathway. Properdin binds to C3b, the C3-convertase of the alternative signalling pathway, and stabilises it, by which the protein stays longer active and as a result cleaves more C3 molecules. This causes an increase in production of C3b and C3a and enhances the marking and destruction of pathogens by the complement system.
Furthermore, properdin is also able to directly bind to pathogenic bacteria, marking it for immune cells and enhancing the complement mediation of cell death and destruction.
Diagnosing the cause of complement defects
The lack or deficiency of protein cleavage is a sign for complement defect. This could be inherited or caused by bacterial infections. Hence its diagnosis focuses on the detection of proteins and its cleavage reaction. In many cases the determination of protein fragments C3 and C4 is sufficient. To capture both the defect and the activation of the system, a stepwise procedure is necessary to analyse all activation pathways and the individual factors.
With which product can Szabo-Scandic support you in the complement diagnostics?
 With the functional complement assays from SVAR you can test all three pathways at once or individually via ELISA:
With the functional complement assays from SVAR you can test all three pathways at once or individually via ELISA:
- WIECOMPL300 WIESLAB® Complement System Screen, ELISA
- WIECOMPLCP310 WIESLAB® Complement System Classical Pathway, ELISA
- WIECOMPLMP320 WIESLAB® Complement System MBL Pathway, ELISA
- WIECOMPLAP330 WIESLAB® Complement System Alternative Pathway, ELISA
Further complement assays (ELISA):
 We also offer a cell-based assay with ready-to-use cells:
We also offer a cell-based assay with ready-to-use cells:
We also have ELISAs for Factor H (CE-IVD) and Factor P (RUO) on offer. Please contact our product management directly for these products.

 Deutsch
Deutsch